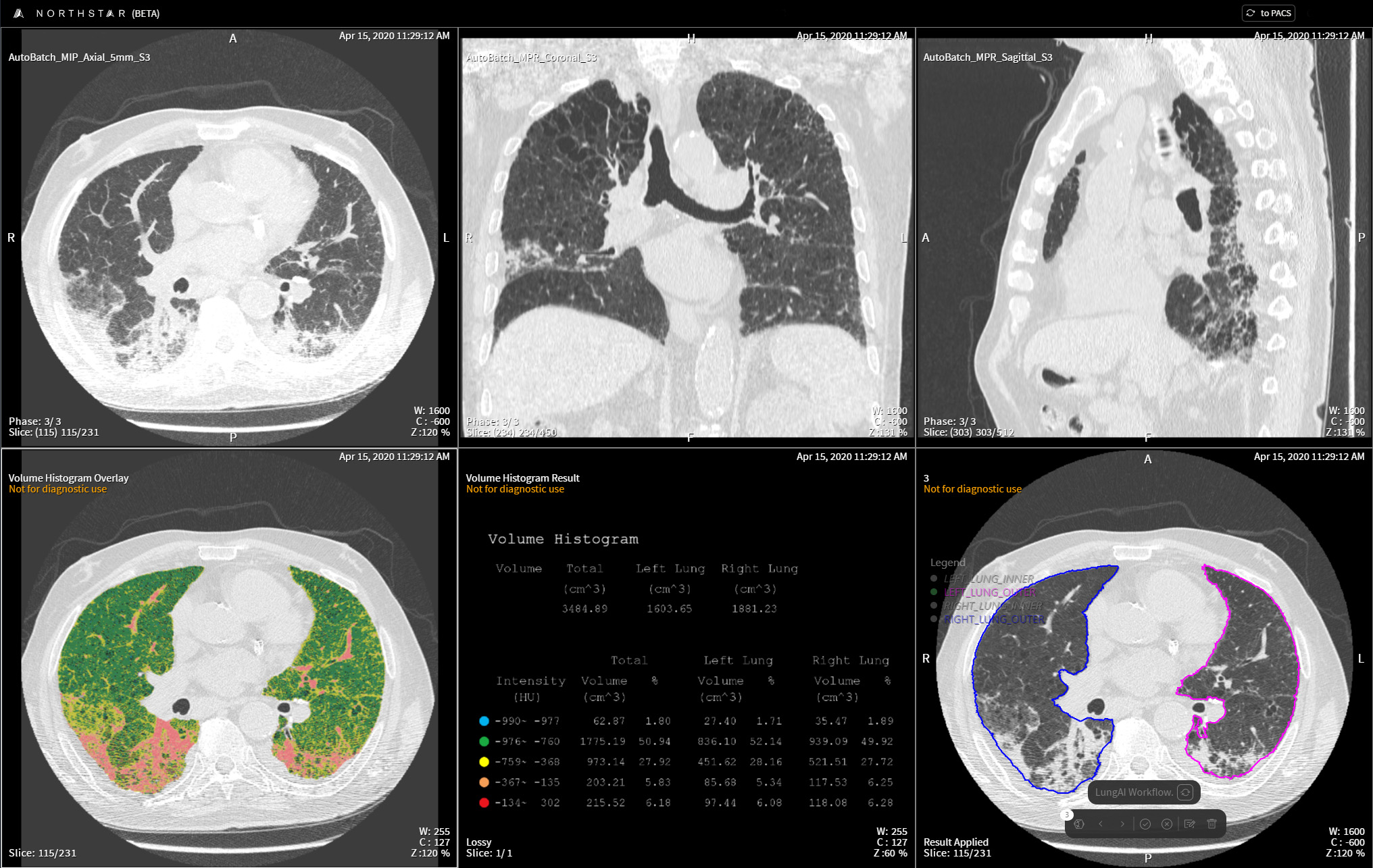
TeraRecon's Lung Density Assessment & Quantification Tools
*The following are important statements regarding the use of this tool:
TeraRecon Headquarters
4309 Emperor Blvd, Suite 310
Durham, NC 27703
Tel: 650.372.1100
Fax: 650.372.1101
info@terarecon.com
All offerings are subject to availability and regulatory clearance, which may vary by country. Please verify product status with your local TeraRecon representative.